First Law of Thermodynamics
First Law of Thermodynamics: Overview
This Topic covers sub-topics such as First Law of Thermodynamics, Mathematical statement of First Law of Thermodynamics, Conclusions from First Law of Thermodynamics, Sign Conventions for Work and, Sign Conventions in Thermodynamics
Important Questions on First Law of Thermodynamics
When mol of a gas is heated at constant volume, temperature is raised from K. If heat supplied to the gas is J, then which statement is correct?
If is the change in enthalpy and the change in internal energy accompanying a gaseous reaction, then
For the above reaction the value of is at . The value of in K.cal is____.(Given ).
One mole of an ideal monoatomic gas is taken round the cyclic process ABCA as shown in fig. Calculate
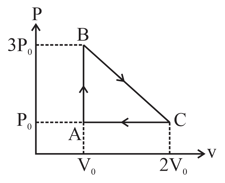
(a) The work done by the gas.
b) The heat rejected by the gas in the path and heat absorbed in the path .
(c) The net heat absorbed by the gas in the path .
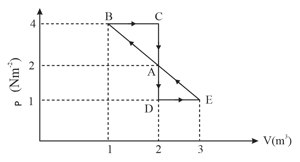
One mole of monoatomic gas is carried along process as shown in the diagram. Find the net work done by gas.
Draw a schematic diagram of a system doing work without changing internal energy? State the law governs it?
How much heat energy is require to raise the temperature of of water by
The enthalpy of combustion of benzoic acid at and pressure is . What is for the reaction ?
When an idea gas in a cylinder was compressed isothermally by a piston, the work done on the gas found to be . During this process about
One mole of an ideal diatomic gas is expanded by heating state-I to against constant pressure . Find the heat supplied in Joule.
(Given that )
Round off your answer to the nearest integer.
Deduce an expression for the work done by a gas in expansion at constant temperature.
Mentioned the sign conventions used in thermodynamics.
The internal energy of a thermodynamic system increases from to when of work is done on the system. In this case, heat is supplied to the system.
Consider two states, and , of a thermodynamic system. Let Path represent a reversible process for going from to , while Path represents an irreversible process for the same. Let the work done, heat change, and entropy change for the two processes be denoted by and , respectively. It is observed that the relation is obeyed. The correct statement is
Mathematical statement of the first law of thermodynamics is
State the Sign conventions and Limitations of First Law of Thermodynamics.
According to the first law of thermodynamics, In special cases the statement can be expressed in different ways. Which of the following is not a correct expression?
What are the sign conventions of heat, work and internal energy.
What is the expression of first law of thermodynamics for adiabatic process?
What is the expression of first law of thermodynamics for isothermal process?
